Background:
Amyloid-mediated organ dysfunction is the main determinant of survival for patients with light chain (AL) amyloidosis. Reducing the production of amyloidogenic light chains with plasma cell-directed therapy is a prerequisite for organ response. Despite achieving a hematologic complete response (hemCR), some patients have enduring organ dysfunction. A better grasp of the mechanisms underlying organ recovery is needed, especially as attention moves to novel therapeutics that trigger removal of existing amyloid deposits, and as graded organ response criteria to gauge improvement more precisely than traditional binary systems are adopted.
Aims:
We designed this study to identify the (1) rates of organ non-response and (2) possible reasons for impaired organ recovery in patients with AL amyloidosis with hemCR after treatment.
Methods:
Patients with biopsy confirmed AL amyloidosis who presented to our center for post-treatment assessment between February 2019 and June 2023 were included if they had major organ involvement (heart, kidney and/or liver) and were evaluable for organ response (Palladini, 2012). Graded cardiac response was assessed by validated criteria (Muchtar, 2022). We excluded patients who received amyloid fibril-targeted antibody therapies. To eliminate the possibility of insufficient hematologic response as a confounder for organ non-response, we focused only on patients with hemCR. Organ response was measured as best response >3 months from hemCR achievement. Reasons for organ non-response were postulated by our multidisciplinary team after extensive workup and review of clinical data.
Results:
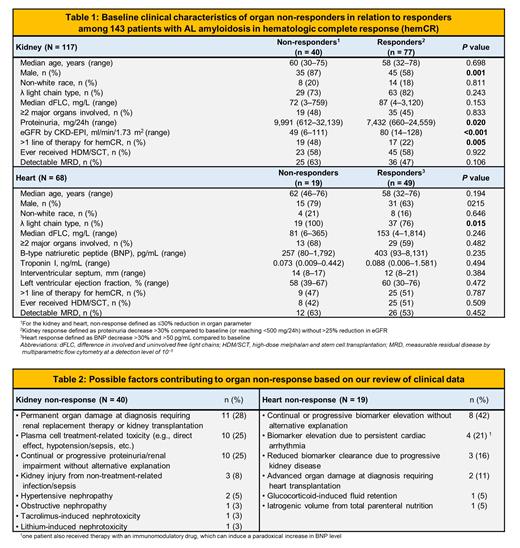
Among 143 patients with hemCR, the median age at diagnosis was 58 years (range 30-78); 94 (66%) were male; and 24 (17%) identified as non-white race. Kidney, heart and liver involvement was present in 117 (82%), 68 (48%) and 17 (12%) patients, respectively; 54 (38%) had ≥2 major organs affected. Final treatment granting hemCR was high-dose melphalan and stem cell transplantation for 49 (34%); bortezomib-based for 37 (26%); daratumumab monotherapy for 31 (22%); daratumumab-CyBorD for 18 (13%); and an immunomodulatory drug for 8 (6%) patients; 58 (41%) required ≥2 lines of therapy to achieve hemCR.
Failure to attain organ response was observed in 40/117 (34%), 19/68 (28%) and 3/17 (18%) of patients with kidney, heart and liver involvement, respectively. Of 42 patients with both kidney and heart involvement, 21 (50%) had concordant responses of both organs; 6 (14%) had concordant non-responses; and 15 (36%) had discordant responses. Among 49 heart responders, 14 (29%) attained CarCR (nadir BNP ≤80 pg/mL); 16 (33%) CarVGPR (>60% reduction); and 19 (39%) CarPR (31-60% reduction); 40/77 (52%) kidney responders attained proteinuria <500 mg/24h.
Clinical features of organ non-responders in relation to responders are reported in Table 1. Notably, kidney non-responders had more advanced disease at baseline, whereas heart non-responders more often had lambda-typic amyloidogenic light chains. Among kidney non-responders, 2 had stable and another 2 had rising proteinuria level; the remaining 36 had improved proteinuria but were categorized as non-response due to >25% worsening of eGFR. Among heart non-responders, 5 had stable elevation of BNP and 14 experienced organ progression (BNP increase >30% and >50 pg/mL); 2 required heart transplantation.
The two most common factors believed to underlie kidney non-response were permanent kidney damage at diagnosis needing dialysis and treatment-related toxicity (Table 2). For 10 (25%) kidney non-responders, there was no apparent explanation, 7 of whom had detectable measurable residual disease (MRD) by multiparametric flow cytometry. Most cases of heart non-response had no apparent explanation; 6 of these 8 patients had detectable MRD.
Conclusions:
Despite effective treatment of the plasma cell clone, a considerable number of patients with AL amyloidosis in hemCR have enduring organ dysfunction. We found that the reasons for organ non-response differed across organ systems. Response was not always concordant for all involved organs, suggesting heterogeneity in organ biology in terms of mechanisms for amyloid-mediated injury and intrinsic ability to repair. Careful scrutiny of reasons for organ non-response is warranted before providing further hematologic treatments, which may be ineffective for organ recovery.
OffLabel Disclosure:
Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Some patients included in this analysis were treated with off-label use of high-dose melphalan and stem cell transplantation; bortezomib; and/or an immunomodulatory drug for AL amyloidosis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal